Keywords: Recurrence, Glioblastoma, Anaplastic astrocytoma, Alisertib, Stereotactic radiation therapy, Phase I clinical trial
ABSTRACT
Background and Purpose: We conducted a phase I trial of alisertib, an oral aurora kinase inhibitor, with fractionated stereotactic re-irradiation therapy (FSRT) for patients with recurrent high grade glioma (HGG).
Materials and Methods: Adult patients with recurrent HGG were enrolled from February 2015 to February 2017. Patients were treated with concurrent FSRT and alisertib followed by maintenance alisertib. Concurrent alisertib dose was escalated from 20 mg to 50 mg twice daily (BID).
Results: Seventeen patients were enrolled. Median follow-up was 11 months. Median FSRT dose was 35 Gy. There were 6, 6, 3, and 2 patients enrolled in 20 mg, 30 mg, 40 mg, and 50 mg cohort, respectively. Only one dose-limiting toxicity (DLT) was observed. One patient in the 20 mg cohort had severe headache (Grade 3) resolved with steroids. There was no non-hematological grade 3 or higher toxicity. There were two Grade 4 late toxicities (one with grade 4 neutropenia and leukopenia, one with pulmonary embolism). One patient developed radiation necrosis (Grade 3). Sixteen patients finished concurrent treatment and received maintenance therapy (median cycles was 3, range 1–9). Overall survival (OS) for all cohorts at 6 months was 88.2% with median survival time of 11.1 months. Progression-free survival (PFS) at 6 months was 35.3% with median time to progression of 4.9 months. The trial stopped early due to closure of alisertib program with only 2 of 3 planned patients enrolled in the 50 mg cohort.
Conclusion: Re-irradiation with FSRT combined with alisertib is safe and well tolerated for HGG with doses up to 40 mg BID. Although no DLT observed in the 50 mg cohort, this cohort was not fully enrolled and maximum tolerated dose (MTD) was not reached. Clinical outcomes appear comparable to historical results.
INTRODUCTION
Recurrence is an inevitable process for most patients with high grade glioma (HGG) despite standard of care treatment. The median progression-free survival for glioblastoma (GBM) was 10.3 months in MGMT-methylated patients, and just 5.3 months for MGMT-unmethylated patients, with a median survival after progression of 6.2 months. There is no consensus for optimal treatment for recurrent HGG. Salvage treatment options include surgery, re-irradiation, chemotherapy/systemic therapy, supportive care only, and more recently, tumor treatment fields (TTFields). Fractionated stereotactic re-irradiation therapy (FSRT) has been well established at our institution for treatment of recurrent HGG. FSRT has been utilized in similar contexts at different institutions with favorable safety and efficacy. Despite these results, there remains significant room for improvement. In order to increase disease control and survival rates, we sought a systemic therapy agent which could enhance the effects of radiation while minimizing overall toxicity.
Alisertib (MLN8237, Takeda Oncology, Cambridge, MA) is a second generation Aurora A kinase inhibitor with anti-neoplastic and radio-sensitization activity. Aurora A is a serine/threonine kinase (AURKA) that regulates mitotic bipolar spindle assembly and centrosome duplication, maturation, and separation. Alisertib MLN8237 sclc is a small molecule that selectively inhibits AURKA by a factor of 200 compared to Aurora B kinase. Overexpression of AURKA has been associated with multiple cancers believed to be driven by chromosomal instability and subsequent aneuploidy. In GBM, overexpression of AURKA is observed, with inhibition of AURKA leading to inhibition of tumor growth as well as chemopotentiation for in vitro and in vivo models. In addition, alisertib has been shown to have significant antitumor activity in bevacizumab resistant in vitro and in vivo GBM models. It has been trialed in both hematological/lymphatic (multiple myeloma, non-Hodgkin lymphoma, chronic lymphocytic leukemia) and solid tumor malignancies (head and neck, breast, small cell and non-small cell lung cancer, gastroesophageal) with a relatively favorable safety profile, with toxicity mostly hematologic in nature. The combination of radiation and AURKA inhibition can lead to an additive response that follows dose, as seen in multiple cell lines, most notably in those with p53 deficiency.
Here we report our results from an open label phase I trial conducted at our institution investigating the safety, tolerability, and preliminary efficacy of alisertib combined with FSRT for recurrent high-grade gliomas in adult patients. This trial is the first of its kind to incorporate radiation and alisertib together in a clinical setting.
MATERIALS AND METHODS
The Thomas Jefferson University institutional review board (IRB) approved this prospective phase I trial which was registered on www.clinicaltrials.gov (NCT02186509). The trial was an investigator-initiated trial (IIT) and sponsored by Thomas Jefferson University (Philadelphia, PA).
Patients
Patients age ≥18 years old, Eastern Cooperative Oncology Group (ECOG) <2, previously histologically confirmed HGG (astrocytic or oligodendroglial supratentorial tumors, World Health Organization grade III or IV) treated with fractionated radiation therapy (≥60 Gy) and now with evidence of recurrence were eligible. Minimal time interval from end of primary radiation treatment and subsequent FSRT start was 3 months. Prior re-irradiation was excluded except for stereotactic radiosurgery (SRS) with V12 <5 cc. Prior treatment with systemic therapy agents including biologics was allowed as long as there was a minimal 2-week washout period. Adequate renal, hepatic, and hematologic function were also required. Informed consent was obtained from all patients with privacy rights always observed.
Trial Design
We utilized a classic 3+3 dose escalation study design to the corresponding alisertib dose level, starting from the initial cohort of 20 mg twice daily to the final cohort of 50 mg twice daily, all with concurrent FSRT. Patients who successfully completed the concurrent treatment would then have a 1-month treatment break before starting maintenance therapy. Study drug would be stopped during the concurrent portion for any dose limiting toxicity (DLT), and during the maintenance portion for disease progression, clinical deterioration, or toxicity.
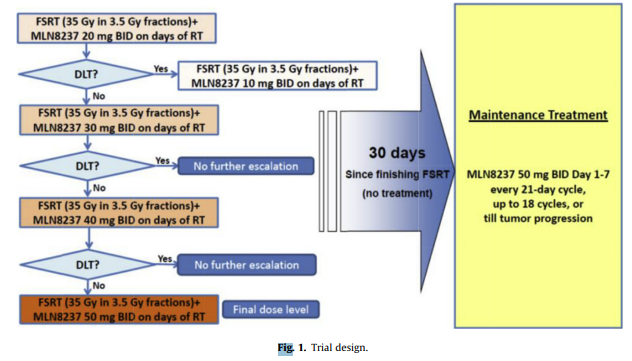
Figure 1. Trial Design.The figure illustrates the treatment schema showing the dose escalation design from 20 mg to 50 mg twice daily concurrent with FSRT, followed by maintenance therapy at 50 mg twice daily for 1 week every 3 weeks for up to 18 cycles.
Radiation Treatment
Treatment planning was carried out with Brain Lab iplan (Brainlab, Munich, Germany), or Eclipse (Varian, Palo Alto, CA). Radiation treatment was delivered with Truebeam STx (Varian) using high definition multileaf collimator and ExacTRAC (Brainlab, Munich, Germany) on board daily imaging guidance. All patients undergoing irradiation were fitted with custom-made Brainlab thermal plastic masks for immobilization. Treatment planning MRI and CT images were obtained and fused. All patients had thin cut (1–1.5 mm) axial post-contrast and T2/FLAIR MRI. The gross tumor volume (GTV) was defined as enhancing tumor on T1 post-contrast MRI. Surrounding edema was not purposely included in the treatment volume. Due to most recurrences are in the prior high dose region, no clinical target volume (CTV) is added to reduce the risk of radionecrosis. The planning target volume was the GTV with minimum margin (0–2 mm per treating physician). Critical normal structures, such as optic nerves, chiasm, and brainstem were also contoured. The radiation planning used dynamic conformal arcs, intensity-modulated radiation therapy (IMRT), or hybrid-arcs (combination of dynamic arcs with IMRT beams). The patients were treated with FSRT to a median planning target volume (PTV) dose of 35 Gy delivered in 3.5 Gy fractions. The dose was reduced to 30 Gy in 3 Gy fractions for large targets (PTV >100 cc), and/or high critical normal structure dose. The constraints for normal critical structures include: brainstem max dose <20 Gy, optic nerve max dose <15 Gy, and chiasm max dose <15 Gy.
Alisertib Treatment
Alisertib was provided by Takeda Oncology. During concurrent phase with radiation, alisertib was administered by mouth concurrently with radiotherapy twice daily at determined dose level for each patient, first daily dose given prior to radiation session. Concurrent phase doses of alisertib were started at 20 mg, then 30 mg, 40 mg, and lastly 50 mg. After a month break, all patients would begin maintenance therapy. Alisertib was administered in cycles of 1 week of treatment every 3 weeks at 50 mg bid, up to 18 cycles or till progression or intolerability.
Assessment of Outcome
Progression of disease was defined by Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Work Group (RANO). MRI brain with/without contrast was obtained between weeks 4–6, then every 2 months for the first year, then every 3 months through the second year after which was obtained every 4–6 months. MRI perfusion was routinely performed as well. MRI perfusion results along with RANO criteria were used to differentiate tumor progression vs radionecrosis.
Toxicity Reporting
Toxicity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0. A DLT was defined as an adverse event (AE) found to be related (definitely, probably, or possibly) to study therapy observed up to 1 month after finishing concurrent treatment. Hematologic DLTs were Grade 4 neutropenia ≥7 days, anemia, thrombocytopenia, or Grade 5 hematologic toxicity. Non-hematologic DLT was Grade 3, 4 or 5 non-hematologic toxicity with exception of Grade 3 nausea, vomiting, diarrhea, dehydration, electrolyte abnormalities, isolated transaminitis not lasting 14 days, acidosis or alkalosis, and alopecia. Symptoms clearly related to the cancer or other pathology (in the opinion of treating physician) and expected sequelae of the neurosurgical procedure or radiosurgery were excluded. All neurological symptoms were considered “treatment related” unless present at study onset, expected sequelae of the neurosurgical procedure, or clearly related to disease progression. AEs were reported to our institution’s IRB, study sponsor, and Data Safety Monitoring Committee (DSMC). All serious adverse events were reported to the FDA.
Statistical Analysis
Our primary objective will be to evaluate the safety and tolerability of the study treatment, as well as determining DLT and the Maximum Tolerated Dose (MTD) of alisertib when combined with FSRT for recurrent HGG. Secondary objectives include estimation of 6-month progression-free survival (PFS), median time to progression (TTP), and overall survival (OS). PFS and OS were measured from date of first day of FSRT.
All evaluable patients underwent statistical analysis. Kaplan–Meier’s with log-rank test was performed for PFS, TTP, and OS. Cox proportional hazard model was performed to evaluate variables affecting clinical outcomes. Analysis was performed on R version 3.5.0.
Eligible patients were screened and enrolled in a 3+3 dose escalation study, with planned maximum of 24 patients. Accrual occurred in two stages, 3 patients at each dose level. If no DLT was experienced by the 3 patients, then the trial moved to the next dose. If at least one DLT was reported at that level, 3 more patients were accrued at that dose. If there were >1 DLT on a given dose level, accrual would have been terminated. No patient received higher dose until the 3 or 6 patients had completed their toxicity evaluation period at the current dose. With this plan, a dose with a 50% or greater probability of causing a dose-limiting toxicity has at most a 12.5% chance of satisfying the conditions for dose escalation after the first 3 subjects and at least a 50% chance of stopping at 3. With the two-stages (3–6) together, there was at most a 17.2% chance of escalation.
RESULTS
We screened 38 patients. Seventeen were successfully enrolled and assigned to study drug. Screening failures included 18 patients who declined to participate and 3 patients who did not meet inclusion criteria.
Of 17 evaluable patients, 11 had GBM and 6 had anaplastic astrocytoma WHO Grade III (AA). It was the first recurrence for 14 (82.4%) patients, second recurrence for two (11.8%) and third recurrence for one. The majority (76.5%) of patients were intermediate risk, based on the Re-RT Risk Score (RRRS), with a median of −0.11 (range −0.47 to 0.29). Time from completion of initial radiation for primary disease to start of FSRT was a median of 12 months (range 4.4–106.2 months). Twelve patients were male, and five were female. Median age at enrollment was 61 years old (range 33–72 years old) with median Karnofsky Performance Status (KPS) of 80 (range 80–90). Six patients had resection prior to alisertib. There were 6, 6, 3, and 2 patients enrolled in the 20 mg, 30 mg, 40 mg, and 50 mg cohorts, respectively. Thirteen patients (76.5%) received the originally designated 35 Gy in 10 fractions for their FSRT dose while four patients (23.5%) received a lower dose at 30 Gy in 10 fractions due to concerns for toxicity to normal structures in the re-irradiated field. The median planning target volume (PTV) was 34.2 cc (range 4.4–117 cc). Of the 17 enrolled patients, sixteen finished concurrent treatment and went on to maintenance cycles (MC). Median number of MC was 3 (range 1–9). One patient (20 mg) never received MC due to DLT during the concurrent phase, while one (20 mg) stopped after the first MC due to persistent neutropenia. The remainder of patients stopped MC due to disease progression and electing salvage treatment, hospice, clinical deterioration, or death.
With median follow up of 11 months, only 2 patients were alive at the time of writing. OS for all cohorts at 6 months was 88.2% with median survival time of 11.1 months. Breaking down by cohort, the median OS for the 20 mg, 30 mg, and 40 mg cohort was 9.4, 16.9, 14.2, and 5.7 months, respectively. Long term survivors (living >12 months after FSRT started) were seen with 2 (33.3%) patients in the 20 mg cohort (14.2 and 13.5 months), four (66.7%) in the 30 mg cohort (20.5, 17.9 months, 16.6 months, and one still alive at 15.4 months), and two (66.7%) in the 40 mg cohort (13.9 and 17.8 months). PFS at 6 months was 35.3% with median TTP of 4.9 months. All but one patient recurred at time of writing. This patient (40 mg), shows no evidence of clinical or radiographic progression at 17.8 months of follow-up, with study drug held after 7 MC due to concerns for deconditioning, and was never restarted nor received subsequent treatment due to lack of evidence of disease. There was no statistically significant survival difference between AA and GBM. Post hoc analysis with Cox proportional hazard and log-rank test of patient and treatment variables including: age >65 years old, PTV >35 cc, gender, histology, alisertib dose, MC >6, FSRT dose, and KPS revealed no statistically significant values for clinical outcomes except for age >65 doing worse with PFS (p = 0.043; log-rank test) and MC >6 doing better with OS (p = 0.049; log-rank test). The latter finding, however, may be more representative of disease progression and the tolerability of the study drug rather than a true response relationship.
Of the 16 patients with recurrence, 11 underwent salvage treatment, 4 received hospice care, and 1 expired before receiving treatment. No measurable response per RANO criteria was observed apart from stable disease or lack of progression. Salvage treatment included BCNU with bevacizumab (two patients), carboplatin (four, 1 had surgery prior and 1 had stereotactic radiosurgery prior 24 Gy), gemcitabine (one), bevacizumab (three of which 1 subsequently had stereotactic body radiation therapy 25 Gy/5 fractions for distal recurrence), and one enrolled on the Toca 5 trial (NCT02414165). Three withdrew from study after recurring while on protocol.
After initial enrollment into the 20 mg cohort, one patient experienced a Grade 3 headache that was considered a DLT which resolved after a short course of steroids. Per protocol, an additional 3 patients were enrolled onto the 20 mg cohort for a total of six patients, which continued through the 30 mg cohort, until deemed safe by the study sponsor, IRB, and the DSMC to continue forward with enrollment of 3 patients per cohort. After successfully accruing to the 50 mg cohort, the drug program was closed to further accrual by Takeda Oncology. The cohort did not completely fill (2 of 3 patients), and thus by definition, the MTD was left undetermined, with no DLT observed in the 40 mg or 50 mg cohorts.
Overall, alisertib was tolerated well. Apart from the initial DLT reported, no other DLT was observed. Acute toxicities (occurring during and up to 1 month after concurrent phase of treatment) included Grade 1 fatigue in 9 (53%) patients, Grade 2 fatigue in 4 (23.5%) patients, Grade 1 anemia in 6 (35.3%) patients, and Grade 2 lymphopenia in 6 (35.3%) patients. There were no acute toxicities higher than Grade 3. The most common acute adverse events included Grade 1 fatigue, Grade 1 anemia, and Grade 2 lymphopenia. Delayed toxicities (occurring after 1 month from the concurrent phase of treatment) included Grade 1 thrombocytopenia in 7 (41.1%) patients, Grade 1 anemia in 6 (35.3%) patients, Grade 2 lymphopenia in 5 (29.4%) patients, Grade 3 lymphopenia in 5 (29.4%) patients, and Grade 1 gait disturbance in 5 (29.4%) patients. There were no Grade 5 toxicities. There were only 2 patients (20 mg) that experienced Grade 4 AE, one with both leukopenia and neutropenia, and one with a pulmonary embolism which was reported with “possible” relation to study drug. One patient developed grade 3 radiation necrosis, which was diagnosed based on MRI and MRI perfusion study and subsequently treated with bevacizumab. The most common delayed AEs were Grade 1 thrombocytopenia, Grade 1 anemia, Grade 2 lymphopenia, and Grade 1 gait disturbance.
DISCUSSION
Our trial is the first of its kind in combining alisertib with radiation as part of protocol in the clinical setting. Our primary objective for determining the safety and tolerability of the study drug was met. There was only one DLT, with no Grade 5 AE in either the acute or delayed setting. We safely and successfully escalated the study drug dose from 20 mg at our initial cohort to 50 mg in our final cohort. Although the MTD was not technically achieved at 50 mg due to the study being closed to further accrual by Takeda Oncology before the 50 mg cohort was filled, no DLT was reported by the 2 of planned 3 patients. The 50 mg bid regimen of alisertib has been concluded as the MTD for several indications. As expected, the more common and severe AE were hematological in nature as described in previous studies. We believe that based on our study results and those of the past, alisertib could be safely administered at 50 mg bid with FSRT for recurrent HGG with careful monitoring of future patients on trial. Officially the MTD was not determined. Additional studies may finalize the appropriate dosing regimen.
Recurrent HGG have been trialed with numerous options including re-resection, re-irradiation, systemic therapy, anti-angiogenesis, and tumor treating fields. Median survival for these trials range from 6.6 to 16 months, which falls within results of our study (11.1 months). At our institution, we previously published studies with FSRT having median OS of 11 and 10.5 months. PFS for alisertib was 35.3% at 6 months, which is higher than those reported for tumor treating fields (21.4%) and the physician’s chemotherapy of choice (15.1%). Our median TTP was 4.9 months which is similar to those seen in the re-irradiation setting. Our study shows that FSRT with alisertib has similar clinical outcomes.
As a phase I trial, our limitation is primarily in the small number of patients inherent to such a design. A phase II trial may elucidate the potential efficacy of alisertib as well as uncover any other toxicity concerns not seen in this phase I trial.
Future directions for the alisertib program are not clear. At time of writing, there were 59 trials registered with ClinicalTrials.gov of which two were actively recruiting (metastatic triple negative breast cancer for adults and rhabdoid tumors for children). It has been the most productive compound from the class of aurora kinase inhibitors, especially in comparison to aurora B kinase inhibitors (barasertib) or pan aurora kinase inhibitors (tozasertib). A phase II trial investigated alisertib for relapsed/refractory B-cell non-Hodgkin lymphoma (NHL), which resulted in a negative study due to overall response rate < 20% and significant myelosuppression, which was akin to the results of another phase II trial for relapsed/refractory aggressive B- and T-cell NHL. Solid tumor indications seem more promising. A phase I trial in endocrine resistant estrogen receptor positive metastatic breast cancer with fulvestrant showed a favorable safety profile. In a phase I trial for metastatic disease from solid tumors in combination with docetaxel, alisertib was well tolerated and demonstrated antitumor activity. The optimal combination, timing, and appropriate indications for treatment with alisertib still need fine tuning. Our study is unique in its combination of radiation treatment with alisertib which may provide potentially more benefit to clinical outcomes than either modality alone.
Our phase I study shows a favorable safety profile for alisertib when used concurrently with FSRT in the setting of recurrent high-grade gliomas. The PFS, TTP, and OS seem similar to historical results for treating this patient population. Although MTD was not achieved due to early closure of the program, further exploration with study drug development in a phase II trial may provide a better sense of clinical outcomes moving forward.