Poloxamer 188 vs. Tween 80: Choosing the Right Surfactant for Biological Formulations
Abstract
Surfactants are used to stabilize biologics. Particularly, polysorbates Tween 20 and Tween 80 dominate the group of surfactants in protein and especially antibody drug products. Since decades drug developers rely on the ethoxylated sorbitan fatty acid ester mixtures to stabilize sensitive molecules such as proteins. Reasons are first excellent stabilizing properties, and second well recognized safety and tolerability profile of these polysorbates in humans, especially for parenteral applications. However, over the past decade concerns regarding the stability of these two polysorbates were raised. The search of alternatives with preferably less reservations concerning degradation and product quality reducing issues leads, among others, to poloxamer 188 also known as Kolliphor P188, a nonionic triblock-copolymer surfactant. This review sums up current knowledge related to the characterization and physico-chemical properties of poloxamer 188, its analytics and stability properties for biological formulations. Furthermore, the advantages and disadvantages as a suitable polysorbate-alternative for the stabilization of biologics are discussed.
Key Words
Poloxamer 188, Kolliphor P188, Pluronic F68, polysorbates, Tween, antibody stability, biopharmaceutical formulation, surfactant, protein stabilization, critical micelle concentration, degradation, parenteral administration, toxicity, tolerability, nonionic triblock-copolymer, polyethylene oxide, polypropylene oxide, monoclonal antibodies, therapeutic proteins.
Introduction
Currently, the biopharmaceutical industry is heavily relying on the use of polysorbates 20 and 80, also abbreviated as PS20 and PS80, as surfactants for the stabilization of protein drugs. These compounds are known as Tween 20 and Tween 80. Surfactants are used to prevent protein aggregation and to stabilize proteins against surface and stress-induced damage. Among the most commonly used surfactants, polysorbates are preferred because of their good stabilizing properties, recognized safety profiles, and regulatory acceptance. However, polysorbates have limitations, including susceptibility to oxidative and hydrolytic degradation, which can generate degradation products that may negatively impact protein stability and product quality.
Polysorbates are complex mixtures of partial fatty acid esters of sorbitol and its anhydrides, copolymerized with approximately 20 moles of ethylene oxide for each mole of sorbitol and its anhydrides. The two most common polysorbates are polysorbate 20, derived from lauric acid with C12 chain, and polysorbate 80, derived from oleic acid with C18:1 chain. These surfactants are polyoxyethylene derivatives of sorbitan fatty acid esters. Due to their amphiphilic nature, polysorbates can interact with both hydrophobic and hydrophilic regions of proteins, providing protection against aggregation and adsorption at interfaces.
Despite their widespread use, polysorbates are susceptible to various degradation pathways. Oxidative degradation can lead to the formation of peroxides and aldehydes, while hydrolytic degradation results in free fatty acids and polyoxyethylene sorbitan. These degradation products can potentially induce protein aggregation, particulate formation, and changes in product appearance. Additionally, enzymatic degradation by host cell proteins such as lipases and esterases can occur during manufacturing processes. These stability concerns have prompted the search for alternative surfactants that offer comparable or superior stabilizing properties with improved chemical stability.
Poloxamers, also known as Pluronics or Kolliphor, represent a class of nonionic triblock copolymers composed of a central hydrophobic chain of polyoxypropylene (PPO), flanked by two hydrophilic chains of polyoxyethylene (PEO). The general structure is PEO-PPO-PEO.Poloxamer 188, commercially available as Pluronic F68 or Kolliphor P188 , has an average molecular weight of approximately 8400 Daltons and consists of approximately 80 percent polyoxyethylene by weight.The poloxamer designation “188” follows a specific nomenclature: the first two digits (18) multiplied by 100 indicate the approximate molecular weight of the PPO core (1800 Daltons), while the last digit (8) indicates the weight percentage of polyoxyethylene divided by 10. These copolymers are essential in pharmaceutical science for their surfactant properties and their ability to stabilize cell membranes.
Poloxamer 188 has been used in various pharmaceutical applications, including as an emulsifying agent, solubilizing agent, and stabilizing agent in both oral and parenteral formulations. It is also known for its ability to protect cell membranes and has been investigated for therapeutic applications in conditions such as sickle cell disease and myocardial infarction. In the context of biopharmaceutical formulations, poloxamer 188 has gained attention as a potential alternative to polysorbates due to its surfactant properties, acceptable toxicity profile, and potentially improved chemical stability.
This review provides a comprehensive evaluation of poloxamer 188 as a surfactant in biological formulations. It covers the physicochemical properties, critical micelle concentration, protein stabilization mechanisms, toxicity and tolerability data, degradation pathways, analytical methods, and regulatory status of poloxamer 188-containing products. The aim is to assess whether poloxamer 188 represents a viable alternative to polysorbates for the stabilization of therapeutic proteins and antibodies.
Surfactants in Protein Formulations
General Considerations
Therapeutic proteins, including monoclonal antibodies, are complex biomolecules that are susceptible to various degradation pathways during manufacturing, storage, and administration. Physical instabilities such as aggregation, precipitation, and adsorption to surfaces can compromise product quality and efficacy. Chemical instabilities including oxidation, deamidation, and hydrolysis can also affect protein structure and function. Surfactants play a critical role in mitigating these instabilities by reducing protein-surface interactions and preventing protein-protein interactions that lead to aggregation.
Surfactants are amphiphilic molecules that consist of both hydrophobic and hydrophilic regions. This dual nature allows them to adsorb at interfaces, reducing surface tension and preventing protein adsorption to container surfaces and air-liquid interfaces. In aqueous solutions above a certain concentration known as the critical micelle concentration, abbreviated as CMC, surfactant molecules self-assemble into micelles, with hydrophobic tails oriented toward the interior and hydrophilic heads exposed to the aqueous environment. These micelles can solubilize hydrophobic molecules and provide additional stabilization to proteins.
The selection of an appropriate surfactant for protein formulations depends on several factors including the specific protein, formulation conditions such as pH, ionic strength, and temperature, the route of administration, and regulatory considerations. Nonionic surfactants are generally preferred for parenteral formulations due to their lower toxicity and better compatibility with biological systems compared to ionic surfactants. Among nonionic surfactants, polysorbates have historically been the most widely used in biopharmaceutical formulations.
Role of Surfactants in Protein Stabilization
Surfactants stabilize proteins through multiple mechanisms. First, they compete with proteins for adsorption at air-liquid and solid-liquid interfaces, thereby reducing protein unfolding and aggregation at these interfaces.During manufacturing processes such as filling, shipping, and handling, proteins are exposed to various stresses including agitation, shaking, and temperature fluctuations that create interfaces where proteins can adsorb and denature. Surfactants preferentially adsorb at these interfaces, creating a protective barrier that prevents protein contact with the interface. This protective action is a cornerstone of biopharmaceutical formulation , ensuring the stability of therapeutic proteins throughout their shelf life.
Second, surfactants can interact directly with proteins, particularly with hydrophobic patches on the protein surface. These interactions can prevent protein-protein associations that lead to aggregation. The hydrophobic portions of surfactant molecules can bind to exposed hydrophobic regions of proteins, effectively shielding these regions from interactions with other protein molecules. However, it is important to note that excessive surfactant concentrations can also lead to protein destabilization through mechanisms such as partial protein unfolding or micellar solubilization.
Third, surfactants reduce the surface tension of the formulation, which minimizes the mechanical stress experienced by proteins during processing and handling. Lower surface tension reduces the formation of foam and bubbles, which are sites where proteins can denature and aggregate. Additionally, surfactants can provide steric stabilization by forming a coating around protein molecules that prevents close approach and aggregation.
The effectiveness of a surfactant in stabilizing proteins depends on its concentration, structure, and the specific formulation conditions. The optimal surfactant concentration is typically in the range of 0.001 to 0.1 percent weight by volume for polysorbates, which is above the CMC but below concentrations that might cause protein destabilization. The surfactant structure, including the length and composition of hydrophobic and hydrophilic regions, determines its adsorption behavior and interaction with proteins.
Limitations of Polysorbates
Despite their widespread use and proven efficacy, polysorbates have several limitations that have become increasingly apparent in recent years. Chemical instability is the primary concern, as polysorbates are susceptible to both oxidative and hydrolytic degradation. Oxidative degradation is initiated by reactive oxygen species and can be catalyzed by trace metal ions such as iron and copper. This process generates peroxides and aldehydes, which are reactive species that can modify proteins through mechanisms such as carbonylation and crosslinking.
Hydrolytic degradation of polysorbates occurs through ester bond cleavage, releasing free fatty acids and polyoxyethylene sorbitan. This degradation is accelerated at extreme pH values and elevated temperatures. The free fatty acids generated can form insoluble particles, particularly in the presence of metal ions, leading to visible particulate matter in the drug product. Additionally, the loss of intact polysorbate reduces the surfactant’s ability to protect proteins, potentially leading to increased protein aggregation.
Enzymatic degradation by residual host cell proteins is another concern, particularly for products manufactured in mammalian cell culture systems. Lipases and esterases from the host cells can hydrolyze the ester bonds in polysorbates, even at very low enzyme levels. This enzymatic degradation can occur during both the manufacturing process and storage, contributing to polysorbate depletion and the accumulation of degradation products.
The degradation products of polysorbates can have several negative impacts on protein formulations. Peroxides and aldehydes can oxidize sensitive amino acid residues in proteins, leading to protein modification and potential loss of activity. Free fatty acids can bind to proteins, causing precipitation or aggregation. Particulate matter formed from degradation products can affect product appearance, increase immunogenicity risk, and potentially cause adverse reactions in patients. These stability issues have prompted regulatory agencies to require comprehensive assessment and control of polysorbate quality and degradation in biopharmaceutical products.
Another limitation is the complexity and heterogeneity of polysorbate preparations. Polysorbates are mixtures rather than single compounds, with variations in fatty acid composition, degree of ethoxylation, and the ratio of different ester species. This heterogeneity can lead to batch-to-batch variability in performance and makes comprehensive characterization challenging. Different suppliers and manufacturing processes can produce polysorbates with different compositions, potentially affecting formulation performance and stability.
Need for Alternative Surfactants
The limitations of polysorbates have created a need for alternative surfactants that can provide equivalent or better protein stabilization while overcoming the stability and quality concerns associated with polysorbates. An ideal alternative surfactant should possess several characteristics including effective protein stabilization at low concentrations, chemical stability under storage conditions, minimal interaction with proteins beyond stabilization, acceptable safety and toxicity profile for the intended route of administration, regulatory acceptability, and compatibility with manufacturing processes and container closure systems.
Several classes of surfactants have been investigated as potential alternatives to polysorbates. These include other nonionic surfactants such as poloxamers, alkyl maltosides, and alkyl glucosides; ionic surfactants such as sodium dodecyl sulfate, though these are generally less suitable for parenteral applications; and amino acid-based surfactants. Among these alternatives, poloxamers, and specifically poloxamer 188, have emerged as promising candidates due to their nonionic nature, proven use in pharmaceutical formulations, acceptable toxicity profile, and potentially superior chemical stability compared to polysorbates.
Poloxamer 188: Structure and Properties
Chemical Structure and Nomenclature
Poloxamer 188 is a synthetic triblock copolymer consisting of a central hydrophobic block of polyoxypropylene flanked by two hydrophilic blocks of polyoxyethylene. The chemical structure can be represented as HO(C2H4O)a(C3H6O)b(C2H4O)aH, where a and b represent the number of ethylene oxide and propylene oxide units, respectively. The nomenclature of poloxamers follows a specific convention where the first two digits multiplied by 100 give the approximate molecular weight of the polyoxypropylene core, and the last digit multiplied by 10 gives the approximate weight percentage of polyoxyethylene.
For poloxamer 188, the designation indicates that the polyoxypropylene core has a molecular weight of approximately 1800 Daltons, and polyoxyethylene constitutes approximately 80 percent of the total molecular weight. The average molecular weight of poloxamer 188 is approximately 8400 Daltons, with approximately 75 to 85 ethylene oxide units and approximately 27 to 30 propylene oxide units. The molecular weight distribution follows a Gaussian distribution with some polydispersity, typical of polymeric materials.
The synthesis of poloxamers involves sequential anionic ring-opening polymerization of propylene oxide followed by ethylene oxide using a hydroxyl-containing initiator. The polymerization is typically catalyzed by base such as potassium hydroxide. First, propylene oxide is polymerized to form the central polyoxypropylene block. Then, ethylene oxide is added to both ends to form the flanking polyoxyethylene blocks. The molecular weight and hydrophilic-lipophilic balance can be controlled by adjusting the ratio of ethylene oxide to propylene oxide and the total number of monomers added.
Poloxamer 188 is also known by several trade names including Pluronic F68, Kolliphor P188, and Lutrol F68. The Pluronic designation uses a letter to indicate physical form at room temperature, with F indicating flake or solid form. Kolliphor is the trade name used by BASF following a rebranding from the previous name Lutrol. These different names refer to essentially the same polymer, though there may be minor differences in purity and specifications depending on the manufacturer and grade.
Physicochemical Properties
Poloxamer 188 appears as a white, waxy solid at room temperature with a melting point in the range of 52 to 57 degrees Celsius. It is highly soluble in water, forming clear solutions at room temperature. The aqueous solubility increases with decreasing temperature due to the inverse temperature-dependent solubility behavior of polyethylene oxide. Poloxamer 188 is also soluble in ethanol and isopropanol but insoluble in hydrocarbons. The high water solubility is attributed to the large proportion of polyoxyethylene in the structure, which can form hydrogen bonds with water molecules.
The hydrophilic-lipophilic balance, abbreviated as HLB, of poloxamer 188 is approximately 29, indicating a highly hydrophilic character. The HLB value is calculated based on the weight percentage of hydrophilic polyoxyethylene relative to the total molecular weight. A higher HLB value indicates a more hydrophilic surfactant. For comparison, polysorbate 20 has an HLB of approximately 16.7, and polysorbate 80 has an HLB of approximately 15, making poloxamer 188 significantly more hydrophilic than the commonly used polysorbates.
The surface activity of poloxamer 188 is lower compared to polysorbates, meaning that higher concentrations are generally required to achieve the same degree of surface tension reduction. The surface tension of pure water at 20 degrees Celsius is approximately 72 millinewtons per meter. Addition of poloxamer 188 at concentrations above the CMC reduces the surface tension to approximately 42 to 47 millinewtons per meter. In contrast, polysorbate 20 and 80 reduce surface tension to approximately 40 millinewtons per meter at lower concentrations.
The viscosity of poloxamer 188 solutions increases with increasing concentration, particularly at concentrations above the CMC where micelles form. Dilute solutions exhibit Newtonian behavior, while more concentrated solutions may show non-Newtonian characteristics. The viscosity also exhibits temperature dependence due to changes in the degree of hydration of the polyoxyethylene blocks and the equilibrium between unimers and micelles.
Critical Micelle Concentration
The critical micelle concentration is a key parameter that characterizes surfactant behavior in aqueous solution. Above the CMC, surfactant molecules self-assemble into micelles, which are dynamic aggregates with the hydrophobic portions shielded in the interior and the hydrophilic portions exposed to the aqueous phase. The CMC of poloxamer 188 has been reported to vary widely in the literature, with values ranging from approximately 0.0055 percent to greater than 10 percent weight by volume, depending on the measurement method, temperature, and solution conditions.
The wide range of reported CMC values for poloxamer 188 reflects the complex aggregation behavior of poloxamers and the challenges in precisely determining the CMC. Unlike small-molecule surfactants that exhibit a sharp transition at the CMC, poloxamers show a more gradual transition from unimers to micelles over a range of concentrations. This behavior is characteristic of polymeric surfactants with high molecular weights and polydispersity.
Surface tension measurements are commonly used to determine CMC, with the CMC identified as the concentration at which the surface tension versus log concentration plot shows a break point. For poloxamer 188, surface tension measurements have yielded CMC values in the range of 0.1 to 0.5 percent weight by volume at room temperature. However, the gradual nature of the transition makes precise determination of the break point challenging.
Fluorescence spectroscopy using hydrophobic probes such as pyrene is another widely used method for CMC determination. This method detects changes in the fluorescence properties of the probe as it partitions into the hydrophobic core of micelles. For poloxamer 188, fluorescence-based methods have yielded CMC values ranging from 0.001 to 0.1 percent weight by volume, generally lower than values obtained from surface tension measurements.
Light scattering techniques, including dynamic light scattering and static light scattering, can detect the formation of micelles based on changes in scattered light intensity. These methods have reported CMC values for poloxamer 188 in the range of 0.01 to 1 percent weight by volume. The sensitivity of light scattering to larger aggregates makes it particularly useful for detecting micelle formation, though the interpretation can be complicated by the presence of polydispersity.
Temperature has a significant effect on the CMC of poloxamer 188. The CMC generally decreases with increasing temperature, which is opposite to the behavior of many small-molecule surfactants. This temperature dependence is related to the dehydration of the polyoxypropylene core at elevated temperatures, which increases its hydrophobicity and promotes micelle formation. The CMC can decrease by an order of magnitude or more as temperature increases from 20 to 37 degrees Celsius.
The presence of salts and other excipients can also affect the CMC of poloxamer 188. Salts generally decrease the CMC by reducing the hydration of the polyoxyethylene blocks and increasing the hydrophobic effect. The effect depends on the type and concentration of salt, with kosmotropic salts having a stronger effect than chaotropic salts. Proteins and other macromolecules can also influence the CMC through various interactions.
For practical purposes in protein formulations, poloxamer 188 is typically used at concentrations in the range of 0.001 to 1 percent weight by volume. This range encompasses the CMC values reported by different methods and ensures that sufficient surfactant is present to provide protein stabilization while avoiding potential issues associated with very high surfactant concentrations.
Temperature-Dependent Behavior
Poloxamers exhibit unique temperature-dependent properties that distinguish them from other nonionic surfactants. The solubility and aggregation behavior of poloxamers are strongly influenced by temperature due to changes in the hydration state of the polyoxyethylene and polyoxypropylene blocks. At low temperatures, both types of blocks are well hydrated, and the polymer is highly soluble as individual unimers. As temperature increases, the polyoxypropylene block becomes progressively dehydrated, increasing its hydrophobicity.
This temperature-induced dehydration leads to the formation of micelles at lower concentrations as temperature increases. The micelles formed by poloxamer 188 are relatively small, with hydrodynamic diameters typically in the range of 10 to 30 nanometers, depending on concentration and temperature. At even higher temperatures and concentrations, poloxamers can undergo further aggregation and phase transitions, including the formation of liquid crystalline phases and gelation.
The cloud point is the temperature at which an aqueous polymer solution becomes turbid due to phase separation. For poloxamer 188, the cloud point is above 100 degrees Celsius at typical formulation concentrations, indicating that the polymer remains soluble over the entire temperature range relevant for pharmaceutical storage and use. This high cloud point is a consequence of the large fraction of hydrophilic polyoxyethylene in the structure.
The temperature-dependent behavior of poloxamer 188 has implications for its use in protein formulations. The decreasing CMC with increasing temperature means that micelles will form at lower concentrations at physiological temperature compared to refrigerated storage temperatures. This could potentially affect the distribution of surfactant between monomeric and micellar forms and influence protein stabilization. Additionally, the temperature dependence must be considered when extrapolating stability data obtained at elevated temperatures to refrigerated storage conditions.
Protein Stabilizing Properties of Poloxamer 188
Mechanisms of Protein Stabilization
Poloxamer 188 stabilizes proteins through similar mechanisms as polysorbates, but with some important differences related to its molecular structure and properties. The primary mechanism is the competitive adsorption at interfaces, where poloxamer 188 preferentially adsorbs at air-liquid and solid-liquid interfaces, creating a protective barrier that prevents protein adsorption and subsequent denaturation and aggregation. The amphiphilic nature of poloxamer 188, with its hydrophobic polyoxypropylene core and hydrophilic polyoxyethylene blocks, enables effective interface coverage.
However, due to the lower surface activity of poloxamer 188 compared to polysorbates, higher concentrations are typically required to achieve equivalent interface protection. Studies have shown that poloxamer 188 concentrations in the range of 0.01 to 0.1 percent are generally needed to provide effective protection against agitation-induced aggregation, compared to 0.001 to 0.01 percent for polysorbates. The specific concentration required depends on the protein, formulation conditions, and the nature and extent of interfacial stress.
Direct interaction with proteins is another potential mechanism of stabilization. The polyoxypropylene core of poloxamer 188 can interact with hydrophobic patches on protein surfaces, similar to how polysorbates interact with proteins. However, the polymer nature and larger size of poloxamer 188 compared to small-molecule surfactants may result in different binding stoichiometry and interaction dynamics. The polyoxyethylene blocks provide steric stabilization, creating a hydrated layer around the protein that prevents close approach and aggregation.
Some studies have suggested that poloxamer 188 may act as a chemical chaperone, stabilizing proteins through preferential hydration and exclusion from the protein surface. According to this mechanism, poloxamer 188 is preferentially excluded from the immediate hydration layer around the protein, creating a thermodynamic driving force that favors the native protein conformation. This mechanism is similar to the stabilizing effect of osmolytes and some excipients.
Comparative Studies with Polysorbates
Multiple studies have compared the protein stabilization efficacy of poloxamer 188 with polysorbates 20 and 80. The results have been mixed, with some studies showing equivalent or superior performance of poloxamer 188 and others showing better performance of polysorbates. The outcomes depend on several factors including the specific protein studied, the type of stress applied, formulation conditions, and the surfactant concentrations tested.
In studies examining protection against agitation-induced aggregation, poloxamer 188 has generally shown comparable or slightly inferior performance to polysorbates at equivalent weight concentrations. However, when concentrations are adjusted to account for differences in molecular weight and surface activity, poloxamer 188 can provide similar protection. For example, studies with monoclonal antibodies have shown that poloxamer 188 at 0.05 to 0.1 percent can provide equivalent protection against stirring-induced aggregation as polysorbate 20 or 80 at 0.01 to 0.02 percent.
For freeze-thaw protection, which involves both ice-liquid interface stress and cold denaturation, poloxamer 188 has shown variable performance. Some studies report good protection at concentrations of 0.1 percent or higher, while others have found polysorbates to be more effective. The efficacy may depend on the protein’s sensitivity to cold denaturation and the specific formulation buffer and pH.
In terms of long-term storage stability, limited comparative data are available. Some studies have reported that proteins formulated with poloxamer 188 showed similar or slightly better stability over months of storage at refrigerated and accelerated temperatures compared to polysorbate formulations. However, comprehensive head-to-head comparisons under various storage conditions are still needed.
An important advantage of poloxamer 188 observed in several studies is reduced protein surface hydrophobicity and lower subvisible particle formation compared to polysorbate formulations. This suggests that poloxamer 188 may interact differently with proteins or provide more effective prevention of partially unfolded protein intermediates that lead to aggregation. However, more research is needed to understand the molecular basis for these differences.
Concentration Optimization
The optimal concentration of poloxamer 188 in protein formulations typically ranges from 0.001 to 1 percent weight by volume, depending on the specific application and protein. This range is higher than the typical range for polysorbates, reflecting the lower surface activity of poloxamer 188. Below the optimal concentration, insufficient protection against interfacial stress and aggregation may occur. Above the optimal concentration, potential adverse effects such as increased viscosity, protein-surfactant interactions that could affect protein structure or activity, and potential toxicity concerns may arise.
A systematic approach to concentration optimization involves testing a range of concentrations under relevant stress conditions, including agitation, freeze-thaw, and elevated temperature storage. The minimum effective concentration that provides adequate protection should be selected to minimize potential adverse effects and cost. Additionally, compatibility with the specific protein, other formulation excipients, and the intended manufacturing process and container closure system should be confirmed.
Some studies have employed orthogonal analytical methods to assess the effects of different poloxamer 188 concentrations on protein stability. These methods include size exclusion chromatography to measure aggregation, light obscuration and microflow imaging to assess particulate formation, differential scanning calorimetry and intrinsic fluorescence to evaluate conformational stability, and bioassays or binding assays to confirm retention of biological activity. The integration of data from multiple analytical methods provides a comprehensive assessment of the optimal poloxamer 188 concentration.
Formulation Considerations
When formulating proteins with poloxamer 188, several factors should be considered beyond the surfactant concentration. The formulation pH can affect both protein stability and poloxamer behavior. While poloxamer 188 is stable and functional over a wide pH range, the protein’s charge state and stability are pH-dependent. The buffer system should be selected to maintain the desired pH and provide adequate buffering capacity.
Ionic strength and the presence of salts can influence protein-surfactant interactions and the CMC of poloxamer 188. Moderate salt concentrations generally decrease the CMC and may enhance surfactant efficacy. However, very high salt concentrations could potentially lead to salting-out effects or other undesired interactions. The specific salt composition should be optimized based on protein requirements and physiological compatibility.
Other excipients commonly used in protein formulations, such as sugars, polyols, amino acids, and antioxidants, should be tested for compatibility with poloxamer 188. Some excipients may synergize with poloxamer 188 to enhance protein stability, while others could potentially interfere with surfactant function. Comprehensive formulation development studies should evaluate the combined effects of all excipients.
The protein concentration itself can affect the optimal surfactant concentration. Higher protein concentrations may require proportionally higher surfactant concentrations to provide adequate protection. Additionally, at very high protein concentrations, such as those used in high-concentration formulations for subcutaneous administration, the rheological properties and potential for protein-protein interactions become important considerations that may influence surfactant selection and concentration.
Toxicity and Tolerability of Poloxamer 188
Preclinical Toxicity Studies
Extensive preclinical toxicity studies have been conducted with poloxamer 188, providing a comprehensive database on its safety profile. Acute toxicity studies in rodents have shown that poloxamer 188 has low acute toxicity when administered orally, intraperitoneally, or intravenously. The lethal dose 50 percent, abbreviated as LD50, values are generally greater than 1000 milligrams per kilogram body weight, indicating low acute toxicity. No significant adverse effects were observed at doses up to several grams per kilogram in acute toxicity studies.
Repeated-dose toxicity studies have been conducted in rodents and dogs with administration durations ranging from several days to several months. These studies evaluated potential effects on various organ systems, clinical chemistry parameters, and histopathology. In general, poloxamer 188 was well tolerated at doses up to several hundred milligrams per kilogram per day. Some studies reported transient effects on liver enzymes at high doses, but these were typically reversible upon cessation of treatment. No significant cumulative toxicity or target organ toxicity was identified in these studies.
Reproductive and developmental toxicity studies have shown no evidence of teratogenicity or effects on fertility at doses up to several hundred milligrams per kilogram per day. Studies in pregnant animals found no adverse effects on embryo-fetal development or postnatal development of offspring. Poloxamer 188 is not expected to cross biological membranes extensively due to its high molecular weight and hydrophilicity, which limits potential for fetal exposure.
Genotoxicity studies, including bacterial reverse mutation tests, chromosome aberration tests, and in vivo micronucleus tests, have all been negative, indicating that poloxamer 188 does not have mutagenic or clastogenic properties. Carcinogenicity studies have not been conducted, but the lack of genotoxicity and the chemical structure and properties of poloxamer 188 suggest low carcinogenic potential.
Immunotoxicity and immunogenicity studies have shown that poloxamer 188 has low potential to elicit immune responses. The polymer is not expected to act as a hapten or antigen due to its synthetic nature and lack of functional groups that typically trigger immune responses. However, as with any excipient, the potential for hypersensitivity reactions should be considered in the context of the final drug product formulation.
Clinical Experience and Human Tolerability
Poloxamer 188 has been used in various pharmaceutical and medical applications for several decades, providing substantial clinical experience with its use in humans. It has been used as an excipient in oral and topical drug products, as a plasma expander, and has been investigated as an active pharmaceutical ingredient for therapeutic applications in conditions such as sickle cell disease, myocardial infarction, and burn injury.
The most extensive clinical experience with intravenous administration of poloxamer 188 comes from clinical trials evaluating its potential therapeutic effects. In studies for sickle cell disease, poloxamer 188 has been administered as continuous intravenous infusions at doses up to 3000 milligrams per kilogram over 48 hours. In trials for acute myocardial infarction, single doses of 300 to 460 milligrams per kilogram were administered as intravenous bolus followed by continuous infusion. These clinical studies have provided valuable safety and tolerability data for poloxamer 188 when administered intravenously at doses far exceeding those that would be encountered from its use as an excipient in biopharmaceutical formulations.
The safety profile from clinical trials has generally been favorable. Most adverse events reported were mild to moderate in severity and did not differ significantly between poloxamer 188 and placebo groups. Common adverse events included headache, nausea, and infusion site reactions, which are typical for intravenous administration of any solution. No serious adverse events directly attributable to poloxamer 188 were identified in these studies.
One notable finding from clinical trials was the observation of increased serum creatinine levels in some patients receiving high doses of poloxamer 188. However, this effect was generally transient and reversible, and the clinical significance remains unclear. It has been hypothesized that poloxamer 188 might interfere with creatinine assays or affect renal tubular secretion of creatinine without actually impairing glomerular filtration. Nonetheless, renal function should be monitored in patients receiving high doses of poloxamer 188.
For use as an excipient in biopharmaceutical formulations, the exposure to poloxamer 188 would be substantially lower than in the clinical trials described above. Typical biopharmaceutical formulations contain poloxamer 188 at concentrations of 0.001 to 1 percent weight by volume. Assuming a typical dose volume of 1 milliliter and a body weight of 70 kilograms, the maximum exposure would be approximately 0.14 milligrams per kilogram, which is several orders of magnitude lower than the doses tested in clinical trials.
Regulatory Acceptance
Poloxamer 188 is included in major pharmacopeias including the United States Pharmacopeia, abbreviated as USP, European Pharmacopoeia, abbreviated as Ph. Eur., and Japanese Pharmacopoeia, abbreviated as JP, with monographs specifying quality standards and test methods. The inclusion in these pharmacopeias indicates regulatory recognition and acceptance of poloxamer 188 as a pharmaceutical excipient.
The regulatory status of poloxamer 188 for parenteral use is well established, with approvals for use in various injectable drug products. It is listed in the FDA Inactive Ingredient Database for multiple routes of administration including intravenous, intramuscular, and subcutaneous. The extensive clinical experience and favorable safety profile support its use as an excipient in biopharmaceutical formulations intended for parenteral administration.
However, it is important to note that the regulatory acceptance and approval of any specific drug product depend on the complete formulation, manufacturing process, control strategy, and clinical data for that product. The use of poloxamer 188 as an alternative to polysorbates in a biopharmaceutical formulation would need to be justified based on formulation development studies, stability data, and potentially comparability studies if replacing polysorbates in an already-approved product.
Degradation and Stability of Poloxamer 188
Chemical Stability
A key advantage of poloxamer 188 compared to polysorbates is its potentially superior chemical stability. The triblock copolymer structure of poloxamer 188, consisting of ether linkages in the polyoxyethylene and polyoxypropylene chains, is generally more resistant to hydrolysis and oxidation than the ester bonds present in polysorbates. Ether bonds are stable under a wide range of pH conditions and are less susceptible to acid- or base-catalyzed hydrolysis compared to ester bonds.
Oxidative degradation of poloxamer 188 is possible, particularly under harsh conditions or in the presence of strong oxidizing agents or radical initiators. The ether linkages can undergo oxidation to form peroxides and undergo chain cleavage, but the rate of these reactions is generally slower than the oxidation of polysorbates. Additionally, poloxamer 188 does not contain unsaturated fatty acid chains like polysorbate 80, which are particularly prone to autoxidation.
Studies comparing the oxidative stability of poloxamer 188 and polysorbates under stressed conditions have shown that poloxamer 188 generates fewer oxidative degradation products. For example, exposure to hydrogen peroxide, metal ions, or elevated temperatures resulted in lower levels of peroxides and carbonyl compounds in poloxamer 188 solutions compared to polysorbate solutions. This improved oxidative stability could potentially reduce the risk of protein oxidation induced by surfactant degradation products.
Hydrolytic stability of poloxamer 188 has been evaluated under various pH conditions and temperatures. The polymer shows excellent stability across the pH range typically used in biopharmaceutical formulations, from pH 5 to pH 8. Even under acidic or basic conditions and elevated temperatures, poloxamer 188 shows minimal degradation. This is in contrast to polysorbates, which undergo significant hydrolysis under acidic conditions, generating free fatty acids.
Enzymatic degradation is not a concern for poloxamer 188 due to the absence of ester bonds that can be cleaved by lipases or esterases. This eliminates one of the degradation pathways that can affect polysorbates during manufacturing in mammalian cell culture systems. The absence of enzymatic degradation simplifies process development and reduces the need for extensive enzyme removal or inactivation steps.
Degradation Products and Analytical Methods
The potential degradation products of poloxamer 188 differ from those of polysorbates. Oxidative degradation of poloxamer 188 can lead to the formation of peroxides, aldehydes, ketones, and low molecular weight fragments resulting from chain cleavage. However, the types and levels of these degradation products are generally lower than those observed for polysorbates under similar conditions.
Analytical methods for monitoring poloxamer 188 and its degradation products in biopharmaceutical formulations have been developed and reported in the literature. Size exclusion chromatography is used to assess changes in molecular weight distribution and detect high or low molecular weight degradation products. The appearance of lower molecular weight peaks can indicate chain cleavage, while high molecular weight shoulders might indicate crosslinking or aggregation.
Reversed-phase high performance liquid chromatography, abbreviated as RP-HPLC, can be used to separate poloxamer 188 from protein and other formulation components and to assess purity. Evaporative light scattering detection or charged aerosol detection are typically used because poloxamer 188 lacks significant UV chromophores. These methods can detect changes in the poloxamer 188 profile over time.
Peroxide determination using colorimetric assays such as the ferrous oxidation-xylenol orange assay, abbreviated as FOX assay, or potassium iodide assays can be used to measure oxidative degradation. These assays detect peroxides that may be present in poloxamer 188 or formed during storage. Peroxide levels should be minimized to reduce the risk of protein oxidation.
Nuclear magnetic resonance spectroscopy, abbreviated as NMR, can provide detailed structural information about poloxamer 188 and detect chemical modifications such as oxidation or chain cleavage. Proton NMR and carbon-13 NMR can identify changes in the polyoxyethylene and polyoxypropylene signals that indicate degradation.
Mass spectrometry methods, including electrospray ionization mass spectrometry and matrix-assisted laser desorption ionization time-of-flight mass spectrometry, can provide molecular weight information and identify specific degradation products. These techniques are particularly useful for characterizing the heterogeneity of poloxamer preparations and detecting changes in the molecular weight distribution.
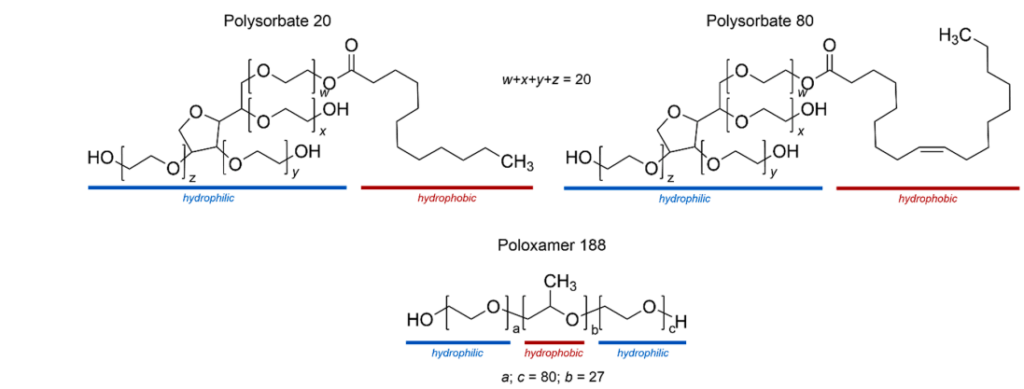
Fig. 1. Structures of main components of polysorbate 20 and 80, as well as poloxamer 188.
Long-Term Stability Studies
Long-term stability studies of biopharmaceutical formulations containing poloxamer 188 have shown favorable results. Several studies have reported that monoclonal antibody formulations containing poloxamer 188 maintained acceptable stability over storage periods of 12 to 24 months at refrigerated temperatures, specifically 2 to 8 degrees Celsius. Parameters monitored included protein aggregation, fragmentation, chemical modifications, potency, and appearance.
Accelerated stability studies at elevated temperatures, typically 25 degrees Celsius and 40 degrees Celsius, are used to predict long-term stability and identify potential degradation pathways. In these studies, formulations with poloxamer 188 have generally shown comparable or better stability than polysorbate formulations. Of particular note is the lower accumulation of subvisible and visible particles in poloxamer 188 formulations compared to some polysorbate formulations, which may be related to the superior chemical stability of poloxamer 188.
Photostability studies evaluating the effects of light exposure on poloxamer 188-containing formulations have shown that the surfactant provides adequate protection against light-induced protein aggregation. Poloxamer 188 itself is stable to light exposure, with minimal degradation under standard ICH photostability testing conditions.
Freeze-thaw stability studies are important for biopharmaceutical products that may be frozen during manufacturing or distribution. Poloxamer 188 has been shown to provide protection against freeze-thaw-induced aggregation in several studies, though the efficacy may depend on the specific protein and formulation conditions. Multiple freeze-thaw cycles can be applied without significant loss of surfactant function.
Compatibility with Manufacturing Processes
Poloxamer 188 has been shown to be compatible with various unit operations in biopharmaceutical manufacturing. It does not interfere with protein expression in mammalian cell culture systems and has even been used as a media supplement to protect cells from shear stress. Poloxamer 188 is compatible with various purification methods including protein A chromatography, ion exchange chromatography, and ultrafiltration/diafiltration.
One consideration for process compatibility is the potential for foaming during filtration and filling operations. Poloxamer 188 has surfactant properties and can generate foam, though generally less than polysorbates at equivalent concentrations. Appropriate process controls and antifoaming strategies can be employed if needed. The polymer is compatible with various types of filters used in biopharmaceutical manufacturing, including sterilizing-grade filters with 0.2 or 0.22 micrometer pore size.
Extractables and leachables from container closure systems may be affected by the presence of poloxamer 188 due to its surfactant properties. Compatibility studies with different container closure materials, including glass vials, prefilled syringes, and various types of stoppers and seals, should be conducted to ensure that poloxamer 188 does not increase extraction of undesirable compounds from these materials.
Approved Biopharmaceutical Products Containing Poloxamer 188
Several biopharmaceutical products approved by regulatory agencies contain poloxamer 188 as an excipient, demonstrating its regulatory acceptability and practical utility. Examples include recombinant human follicle-stimulating hormone products such as Bemfola and Gonal-f, which are used for ovulation induction and contain poloxamer 188 as a stabilizing agent. These products have been on the market for many years with established safety and efficacy profiles.
Gene therapy products including Luxturna, a recombinant adeno-associated virus for treatment of inherited retinal dystrophy, and Zolgensma, an onasemnogene abeparvovec for treatment of spinal muscular atrophy, both contain poloxamer 188 in their formulations. These products represent cutting-edge biopharmaceuticals and demonstrate the suitability of poloxamer 188 for stabilizing sensitive biological products including viral vectors.
Monoclonal antibody products containing poloxamer 188 include Enspryng, a satralizumab formulation for neuromyelitis optica spectrum disorder; Gazyvaro, an obinutuzumab formulation for chronic lymphocytic leukemia and follicular lymphoma; and Hemlibra, an emicizumab formulation for hemophilia A. These products contain poloxamer 188 at concentrations ranging from 0.01 milligrams per milliliter to 8 milligrams per milliliter, equivalent to approximately 0.001 to 0.8 percent weight by volume.
The successful development and approval of these products containing poloxamer 188 provide proof of concept that poloxamer 188 can serve as an effective alternative to polysorbates in biopharmaceutical formulations. The experience gained from these products can inform the development of new formulations using poloxamer 188.
Conclusion and Future Perspectives
Poloxamer 188 represents a viable alternative to polysorbates as a surfactant for stabilizing proteins in biopharmaceutical formulations. Its chemical structure, based on ether linkages rather than ester bonds, provides superior chemical stability compared to polysorbates, with reduced susceptibility to oxidative and hydrolytic degradation. The absence of ester bonds also eliminates concerns about enzymatic degradation by residual host cell proteins during manufacturing.
The protein stabilization mechanisms of poloxamer 188 are similar to those of polysorbates, involving competitive adsorption at interfaces and potential direct interactions with proteins. While the surface activity of poloxamer 188 is lower than that of polysorbates, requiring somewhat higher concentrations, effective protein stabilization can be achieved at concentrations in the range of 0.001 to 1 percent weight by volume. Comparative studies have shown that poloxamer 188 can provide equivalent or superior protection against various stresses including agitation, freeze-thaw, and storage-related degradation.
The safety and tolerability profile of poloxamer 188 is well established through extensive preclinical studies and clinical experience. The polymer has low acute and chronic toxicity, no evidence of genotoxicity or reproductive toxicity, and is well tolerated when administered parenterally at doses far exceeding those encountered from excipient use. The regulatory acceptance of poloxamer 188 is demonstrated by its inclusion in major pharmacopeias and its use in multiple approved biopharmaceutical products.
Despite these advantages, some considerations and limitations should be noted. The lower surface activity of poloxamer 188 compared to polysorbates means that higher concentrations are generally needed, which could impact formulation costs and potentially increase viscosity. The temperature-dependent behavior and the wide range of reported CMC values reflect the complex aggregation behavior of polymeric surfactants and necessitate careful formulation optimization. Long-term stability data and real-world experience with poloxamer 188 in biopharmaceutical formulations are still accumulating, though the available data are encouraging.
Future research directions include further mechanistic studies to understand protein-poloxamer interactions at the molecular level, development of predictive models for optimal poloxamer 188 concentrations based on protein and formulation properties, comprehensive comparability studies for existing products to facilitate replacement of polysorbates with poloxamer 188 where appropriate, investigation of combination approaches using both poloxamer 188 and other stabilizing excipients, and exploration of other poloxamer grades with different molecular weights and hydrophilic-lipophilic balances for specific applications.
The identification and validation of analytical methods for comprehensive characterization of poloxamer 188 and its potential degradation products in complex biopharmaceutical formulations remain important. Harmonized testing strategies and acceptance criteria would facilitate regulatory submissions and product approvals. Additionally, the development of high-purity, well-characterized grades of poloxamer 188 specifically designed for biopharmaceutical use would be valuable.
In conclusion, poloxamer 188 offers a promising alternative to polysorbates for protein stabilization in biopharmaceutical formulations, with potential advantages in chemical stability and reduced degradation-related quality issues. While polysorbates will likely remain important excipients in many products, poloxamer 188 provides formulators with an additional tool in their toolbox for developing stable, safe, and effective biopharmaceutical products. The decision to use poloxamer 188 versus polysorbates should be based on comprehensive formulation development studies, considering the specific protein, intended use, manufacturing process, and regulatory strategy for each product.